How to cite this article:
Conde, S. (2022). Crononutrición: efecto de la hora de la ingesta en el metabolismo de los
nutrientes. MLS Health & Nutrition Research, 1(2), 104-122
CRONONUTRICIÓN: EFECTO DE LA HORA DE LA INGESTA EN EL METABOLISMO DE LOS
NUTRIENTES.
Date received: 08/03/2022 /
Date reviewed: 14/05/2022 /
Date accepted:
03/08/2022
Abstract. Nowadays the metabolic variations are one of the most suffered illness in
the world. Therefore, the investigation of the study about the influence of intake time in the
metabolism of a nutrient, it is gaining importance for the development and applications on new
treatments as far as these diseases are concerned. This bibliographic review, through an in-depth
bibliographic search in different databases, has allowed us to obtain several files, documents,
articles and researches that have been used for the analysis, development and execution of the
current article. The glucose molecule has more persistent levels in the afternoon versus the
morning, due to the decreased insulin activity through the day. Most lipids show their highest
levels in the afternoon. Regarding proteins, more study is needed for their knowledge in this
appearance. More research is required to obtain a better conclusion. Even so, it can be concluded
that the time of intake is a factor that affects the rhythmicity of metabolic processes, interfering
and modifying the activity and response of nutrients.
keywords: chronobiology, metabolism, nutrients, timing, physiological variations.
Resumen. Las alteraciones metabólicas suponen hoy en día una de las afecciones más
padecidas en todo el mundo. Es por ello que la indagación en el estudio sobre la influencia de la
hora de la ingesta en el metabolismo de un nutriente, es de gran importancia para el desarrollo y
aplicación de nuevos tratamientos en lo que a estas enfermedades respecta. Mediante esta revisión
bibliográfica, a través de la búsqueda bibliográfica profunda en diferentes bases de datos, se han
obtenido diversos archivos, documentos, artículos y estudios que han servido para el análisis,
desarrollo y ejecución del vigente artículo. La molécula de la glucosa presenta niveles más
persistentes en la tarde versus la mañana, debido a la disminución de la actividad de la insulina
con el avance del día. La mayoría de los lípidos presentan sus niveles más altos en la tarde. En
cuanto a las proteínas se necesita más estudio para su conocimiento en este aspecto. Se requiere de
más investigación para poder obtener una conclusión más exacta. Aun así, se puede concluir en que la
hora de la ingesta es un factor que afecta en la ritmicidad de los procesos metabólicos,
interfiriendo y alterando la actividad y respuesta de los nutrientes.
Palabras clave: cronobiología, metabolismo, nutrientes, timing u hora de la ingesta,
alteraciones fisiológicas.
Introduction
We are at a time in history in which metabolic disorders of obesity and diabetes crown the list of diseases
most suffered within the human collective. The number of affected people is around 1 billion worldwide (1).
Recent research on the human genome has discovered a possible treatment that can substitute and/or complement
current therapies for these alterations. Which, for different reasons (economic, lack of resources, scope,
etc.), are not 100% effective. We are talking about Chronobiology, a new science that studies the
rhythmicity of physiological processes (2-5).
The main factor of these alterations is the process of eating. The results of which are usually given in
terms of what and how much we eat. However, new studies differ in this, highlighting the existence of
another factor that could participate or be the cause of this. This new factor would be determined by the
time of nutrient intake (6,7).
This is supported by the possible correlation between the metabolic health of the human body and the time at
which a particular macronutrient is ingested, regardless of the total amount of total calories or the food
as a whole. For example, glucose tolerance is highest in the morning, gradually deteriorating as the day
progresses into the evening. This aspect suggests that not only will a response be generated depending on
the type of food or amount of it that we consume, but also the time of day in which we do it will be a
factor that will influence this physiological response in terms of the availability of the nutrient or
nutrients (1,6,8).
All these response processes are the responsibility of the study of chronobiology, which is important for the
understanding of their mechanism of action. In this way, circadian rhythms regulate all these processes
through a synchronization mediated by the known clock genes or circadian clocks. It is composed by a
hierarchical gear headed by the central clock located in the suprachiasmatic nucleus (SCN) of the brain,
which dominates and controls the peripheral clocks located in other organs such as the liver, muscles, or
adipose tissue. While the NSQ is primarily mediated by environmental factor such as light and darkness, the
rest of the peripheral clocks are mediated by several factors, of which food is one of the most important.
In other words, the what, the how much, but, above all, the when, are decisive when it comes to avoiding
disruptions with the NSQ and thus maintaining homeostasis and avoiding metabolic alterations (1,3,8-10).
With all this, it is possible to speculate on the relationship between circadian rhythms and metabolic
function. In such a way that an alteration in each of them entails reciprocal repercussions. An important
role in this is played by the binomial: type or class of macronutrient and time of day when it is ingested.
Therefore, the understanding of the metabolic physiology schedule would serve as a gateway to behavioral
interventions in lifestyle and/or therapies to treat metabolic diseases (1,9).
Method
For the elaboration of this article, a systematic review of the existing scientific literature was carried
out through a bibliographic search, following a defined strategy. Through the use of different online
databases, terms related to the topic, filters (year, type of publication, relevance, author/s, etc.), a
wide selection of documents was compiled, identifying and choosing those with useful information and data
for its development. The main sources and databases used were those related to the field of health that
provided high quality information and scientific evidence, and gave rise to the contrast of ideas. Of a
total of 35 articles used, the main database with the greatest contribution was Pubmed, with a total of 31
articles. In contrast, DOAJ and Google Scholar only contributed a total of 3 articles each, as well as
Cochrane Library and LILACS only provided 1 article each. Temporal publication ranges were established in or
after 2010. The most used terms were: Chronobiology, Chronobiology and metabolism, Chronotype, nutrients,
Circadian physiology, Circadian system architecture, Transcriptional architecture as circadian system,
Central and peripheral clocks, Meal timing health effect, Glucose postprandial response morning evening,
Diurnal glucose and fat levels, Diurnal protein levels, Plasma triglycerides and glucose, diabetes,
Nutrients levels circadian clock health.
Chronobiology in nutrition: chrononutrition
Throughout history, living beings have been forced to adapt to a cyclical and changing world. This has
originated the presence of an adaptive rhythmicity in the physiology of organisms with 24-hour periods
marked mainly by daily light-dark cycles but also by other factors such as food (1, 4, 11, 12).
This rhythmicity is capable of synchronizing, coordinating, and regulating physiological processes (neuronal,
endocrine, metabolic, behavioral, etc.) in the face of fluctuations or variations in time caused by external
and internal factors. Adaptation produced thanks to the presence of genes, known as clock genes or
biological clocks, which allow the body to anticipate any event that influences any of these processes. This
mechanistic and regulatory system is the field of study of chronobiology. This science was first described
in 1729 but was not recognized until the twentieth century through the contributions of the Nobel Prize
winners Jeffrey Hall, Michael Rosbash, and Michael Young through their research on fruit flies (4, 13, 14).
From the hand of chronobiology comes chrononutrition. An emerging discipline subject to it but from a food
point of view. This focuses on the study of the relationship between circadian rhythms and the metabolic
process of food. The clock genes involved in these aspects are located in the main organs involved in
metabolism, namely the liver, pancreas, adipose tissue, and muscle (4, 15).
Architecture of the circadian system
The light-dark binomial generates cycles or approximate rhythms ("circa") of 24h ("diem") in coordination
with the clocks to provide the internal, temporal homeostasis of the organism with the outside. This
mechanism is generated from a feedback loop of gene expression in the phases of cellular transcription and
translation ordered by the central clock. This mechanism will be discussed later (1,16,11).
The central or master biological clock is the one that predominates over the rest of the clocks. It is
located in the anterior part of the hypothalamus, specifically in the interior of the NSQ. Its function lies
in the generation, regulation, and maintenance of all circadian rhythms based on orders transmitted to the
other clocks, the "peripheral clocks" or "oscillators," located in the other tissues, thus allowing
synchronization between the two. While the central clock is determined by the action exerted on it by the
environmental factor of light and darkness, the peripheral clocks are driven not only by the orders of the
central clock but also by external factors such as food or fasting (Figure 1) (3,8,9).
Figure 1. Architecture of the circadian system. Central and peripheral clock mediated by external and
internal factors (3)
Note: Taken from Poggiogalle et al., (2018)
Circadian rhythms
Circadian rhythms are sequenced 24-hour cycles that coordinate all physiological and behavioral processes of
an organism by synchronizing the central and peripheral clocks in response to environmental signals. These
signals are known as Zeitgebers, with sunlight having the greatest effect in mammals, followed by others
such as temperature, feeding/fasting, activity/rest, social cues, etc. (9, 10, 15-17).
This rhythmic succession of actions in living beings makes it possible for it to anticipate any Zeitgeber or
change in the environment (food/metabolism or hunter/prey interaction, DNA damage, etc.), thus preparing its
organism to make decisions at the cellular and behavioral level in order to be as effective as possible,
maintain homeostasis with the surrounding environment, and survive (10,15,16).
Each individual presents a predisposition or preference for a type of active or rest cycle, this is known as
chronotype or circadian preference. In this sense, we find morning or early chronotypes, more active during
the first hours of the day, intermediate and evening or late, more active towards the last hours of the day
(8,10).
Biological clocks or clock genes: a hierarchical system
Biological clocks or clock genes are endogenous timers present in most cells, responsible for genetically
encoding the expression of proteins through a self-regulated feedback loop of transcription and translation,
generating an internal time in them of 24 hours, with or without the presence of external signals. In other
words, they are those whose function resides in the genetic codification and production of circadian rhythms
(16,18).
There are two types of clocks: central and peripheral. Together, they form a network of connections creating
a unique, organized, and sophisticated hierarchical system (Figure 2) (9,16).
Figure 2. Clock output from the central clock to the peripheral cclocks entrained by the light and power
Zeitgebers (9). (RHT; Retinohypothalamic tract, SCN; Suprachiasmatic nucleus, Nm-SCN; non SCN brain
cloks).Note: Taken from Oosterman et al., (2015)
Central or "master" clock: suprachiasmatic nucleus
El reloj central o “maestro” se encuentra ubicado en el interior de cada neurona que conforma el NSQ. Su
función es similar a la de un marcapasos de tipo endógeno; creando, sustentando y regulando ritmos
circadianos de primera orden hacia relojes periféricos del resto de tejidos como respuesta ante la
información recogida desde el exterior ante el estímulo de luz-oscuridad (9,16,17).
In this sense, light is captured by the receptors present in the retina and projected in the NSQ through the
retinohypothalamic tract (RHT), producing the secretion of neurotransmitters that activate certain
substances that stimulate the receptors of the neurons present in the NSQ, ending in the regulation of the
genes that encode for the proteins related to these circadian mechanisms. This signal, transformed into
information, is sent to the rest of the peripheral clocks by means of behavioral, autonomic, neuronal, and
endocrine pathways, so that they can be activated or deactivated (9,15,16,19).
Peripheral clocks: tissue organization
They are semi-independent clocks present in most cells of the rest of the tissues of the main organs involved
in vital functions (liver, pancreas, intestine, stomach, heart, lungs, muscle, etc.) (Figure 1). A priori,
they reestablish their phases of gene expression by means of the signals ordered by the NSQ. The aim of the
NSQ with the peripheral clocks lies in their coordination, activating or inhibiting them. However, their
expression can also be restored by other external signals such as fasting or feeding. Therefore, it can be
pointed out that this control does not occur strictly in most cases, but in some cases, it presents certain
autonomy as in the case of the liver (1,9,16,20).
Molecular mechanism of the circadian clock
The clock mechanism in mammals functions through the joint action of two intertwined feedback loops of the
molecular phase of transcription-translation (TTFL), thus generating 24-hour gene oscillations. For this
feedback to function, the action and dominance of a series of proteins acting on the TTFL and the
corresponding genes is required (1,11,16,18).
On the one hand, there are the CLOCK and BMAL-1 proteins, subunits of the basic heterodimeric transcription
factor Helix-loop-helix (bHLH) Per-Arnt-Sim (PAS), which act as activators (positive product) and are
regulated by the period Per (Per1, Per2, and Per3) and cryptochrome Cry (Cry1 and Cry2) genes. On the other
hand, PER and CRY proteins, which act as repressors (negative product). And finally, kinases (CKIα, CKIδ,
and CKIε) and phosphatases (PP1, PP5), which regulate the localization and stability of the previous
proteins (3,9,16).
Briefly, the mechanism consists of the following: the activator proteins, CLOCK and BMAL-1; they dimerize
forming the CLOCK:BMAL-1 complex (active during the day), which binds to regulatory sequences known as EBox
(5′-CACGTG-3 ') to activate the transcription (in the afternoon) of the three Per genes and the two Cry
genes. (7,18). In the evening, PER and CRY proteins heterodimerize in the cytoplasm and translocate to the
nucleus to interact with the CLOCK:BMAL-1 complex. Once the degradation of PER and CRY by the action of
ubiquitins has begun, the action of the CLOCK:BMAL-1 complex is gradually reduced until it disappears, and
the cycle begins again with the characteristic periodicity of 24 hours (Figure 4) (3,5,9,16,18)
(3,5,9,16,18).
On the other hand, there are metabolic transcription factors that regulate the transcription of cellular
clock mechanism elements through competition for the RORE binding site. These are known as REV-ERBα and
RORα. REV-ERBα is an adipogenesis-regulated factor that inhibits BMal-1 by binding to RORE. And RORα, a
factor also involved in lipid homeostasis which, in contrast to the former, activates BMAl-1 upon binding to
RORE (Figure 4) (5,9).
Figure 4. Network of the molecular mechanism of the circadian clock (5).
Note: Taken from
Maury et al (2019).
Metabolic pathways, major metabolites, and their integration with the circadian clock
Nutrient metabolism works through two main pathways: the anabolic, which acts in the presence of food, and
the catabolic, which acts in its absence. Since the body's energy needs vary according to the degree and
type of activity and the time of day, both pathways take turns without actively participating at the same
time thanks to circadian synchronization (22,23).
In order to maintain energy homeostasis and thus a functional and effective metabolism, circadian clocks must
maintain a reciprocal synchronization with the liver, its metabolic pathways, and metabolites. In this way,
processes necessary for this are enabled and occur. For example, the storage of nutrients during feeding
phases in order to be able to use these stored energy reserves later during fasting phases (1,15).
In addition to the evidence of the presence of clocks in the metabolism, several studies show that mere
disruptions in the metabolic cycles of different metabolites, such as off-time intake or high intakes of a
particular nutrient at a particular time of day can damage the activity of the NSQ and thus the overall
synchronization causing metabolic disturbances. This is known as circadian misalignment. That said, it is
important to take into consideration both the timing and the composition of the food (1,3,9).
Carbohydrates and circadian synchronization
Glucose, the main energy substrate for the organism, presents a series of variations in its plasma levels
throughout the day, and it is of great importance to keep them within normal ranges. To ensure homeostasis
and avoid circadian misalignment or desynchronization, with its possible health effects, clocks and
metabolism are synchronized by working on glucose detection and signaling systems (insulin, glucagon,
somatostatin, etc.) and their transformation processes (9,22).
Anabolic and circadian carbohydrate pathway
Within the anabolic glucose pathway, the main components that show a rhythmicity in their activity during
feeding periods are the following. Firstly, the GLUT2 transporter and GCK show their highest peaks of action
during meals. Secondly, insulin, through a signaling cascade, activates glycogenesis by inhibiting glycogen
synthase kinase (GSK3) and releasing glycogen synthase (GS). GSK3 is characterized by the ability to act on
some circadian system clocks causing alterations; for example, it affects the stability of REV-ERB. Thirdly,
the O-GlcNac protein, which is involved in the degradation, or ubiquitination, of the clock components PER,
CLOCK, and BMAL-1 (Figure 5) (1,24).
Figure 5. Cascade of glucose and insulin metabolic processing by circadian regulation (1).
Note:
Taken from Satchidananda Panda (2016).
Catabolic and circadian carbohydrate pathway
Within the catabolic pathway, which is active during periods of fasting, the glucose metabolic pathway is
also influenced by the circadian mechanism. Since the circadian system and metabolism mobilize glucose from
the tissues to obtain energy, the circadian system and metabolism mobilize glucose from the tissues to
obtain it. In this way, the NSQ signals the hormone glucagon, which binds to receptors (G protein and
adenylate cyclase) that activate protein kinase A (PKA), finally promoting the degradation processes to
obtain the product in question. This PKA phosphorylates activating the response towards cyclic AMP (CREB) to
bind to CRE, activating the transcription steps of PER1 clock components and several glucogenic promoters.
Finally, the role of CRY1 as a stabilizer against the effect of nutrient deficiency should be noted. It
inhibits PKA when it acts negatively on G protein or adenylate cyclase. On the contrary, it is degraded by
AMP-activated kinases once AMP reaches high concentrations induced by prolonged fasting (Figure 6)
(1,25,26).
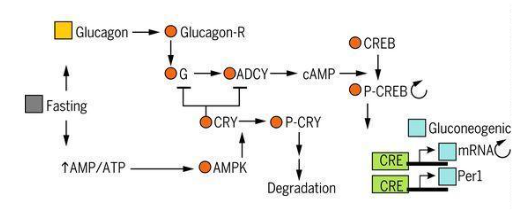
Figure 6: Clock coordination and power signals (1).
Note: Taken from Satchidananda Panda (2016)
Lipids and circadian synchronization
As in the glucose molecule, fatty acid metabolism also presents circadian rhythms in response to feeding or
fasting states. Promoting in the former the activation of the anabolic pathways of lipogenesis and in the
latter the catabolic pathway of β-oxidation (1,15).
Anabolic and circadian lipid pathway
The anabolic pathway of lipogenesis, activated by feeding begins with the exit of acetyl-CoA (AcCoA) from the
mitochondria into the cytosol. Once there, it is carboxylated by the enzyme acetyl-CoA carboxylase (ACACA)
to give rise to the product Malonyl-CoA. ATP citrate lyase (ACLY), the enzyme in charge of acetyl-CoA
synthesis, shows its highest peak expression during this stage, thus showing daily rhythms. In addition, the
entry of fatty acyl groups into the cell by carnitine palmitoyl transferase (CPT) 1 and 2 decreases
catabolic activity. Once high levels of Malonyl-CoA are produced, a response that inactivates CPT is
generated, thus ending lipogenesis (Figure 7) (1,27-30).
Catabolic and circadian lipid pathway
The catabolic pathway of β-oxidation, activated in the absence of food during periods of fasting, begins its
activity by inducing AMPK to phosphorylate ACACA, thus interrupting the anabolic pathway. In contrast, as
noted above, CPT1 and CPT2 decrease the rate of β-oxidation activity by introducing fatty acyl groups into
the cell (Figure 7) (1).
Figure 7: Cascade of the lipid metabolic process through circadian regulation (1)
Note:
Taken from Satchidananda Panda (2016).
Proteins and circadian synchronization
In the same way as the previous metabolites, protein metabolism presents rhythms in response to feeding and
fasting processes with the activation of its main pathways. In this case, the anabolic pathway is activated
to synthesize and store proteins, and the catabolic pathway for gluconeogenesis, formation of active
molecules or release of ammonia for the formation of urea (1,28).
Anabolic and circadian protein pathway
After the feeding phase, the insulin kinase receptor (AKT) activates the mTOR-S6 protein translation pathway.
In turn, AKT and another protein-coding enzyme (S6K1), phosphorylate by recruiting BMAL-1 to promote
translation. This rhythm is of utmost importance for the synthesis of some proteins vital for liver function
such as albumin, proteins of the complement pathway, retinol binding protein or transthyretin (Figure 8) (1)
(1).
Figure 8. Cascade of the metabolic process of amino acids through circadian regulation
(1)
Note:
Taken from Satchidananda Panda (2016).
Catabolic and circadian protein pathway
During the fasting phase, circadian rhythms regulate and activate the KLF15 transcription factors present in
hepatocytes and myocytes. These factors modulate the activity of the enzymes responsible for mobilizing
amino acids from the muscle to the liver for subsequent utilization in gluconeogenesis and ammonia
production for the urea cycle (1,31).
Nutrients and time of intake or timing, health implications
The time of the meal, also known by the term "fashionable timing" and its frequency, are two factors that
notoriously influence the metabolic processes of the main energetic (glucose and lipids) and plastic
(proteins) components of the organism, and consequently, on the synchronization that they maintain with the
circadian system (5,6,32). In this way, these processes manifest throughout the day variations in the peak
of expression of their activity being, in some cases, greater in the first hours and, in others, towards the
last hours of the day. Therefore, the consumption of these components outside the stages corresponding to
their greater tolerance can generate metabolic alterations, as well as circadian disruptions, leading to
diseases such as dyslipidemia and/or diabetes (3,5,6,19,32).
Effects of Nutrients and Time on Health
Understanding the roles of nutrients at each hour of the day within the circadian system brings us closer to
the development of quality health. For as mentioned above, the circadian regulation of these components
involves the maintenance of many of the processes derived from metabolic physiology. Where simple and
minimal misalignments can generate a long list of pathologies, such as glucose intolerance, insulin
resistance, obesity, dyslipidemia, heart disease, chronic inflammation, liver disease, increased risk of
cancer, and even muscle problems (1,3,6,15).
A bad carbohydrate intake plan leads to an imbalance of carbohydrates. This affects more acutely on people
whose tolerance is weaker, as is the case of people with type 2 Diabetes Mellitus. It has been observed that
a high nocturnal intake of carbohydrates in this group, and especially in people with obesity, produces
severe hyperglycemia of long duration. This is also transferred to pre-diabetic people, causing them to
become diabetic during these periods and suffer the same effects. All this is due to the deterioration in
the tolerance of this nutrient because of a poor response of insulin activity during this period of the day.
Therefore, its intake at night should be reduced, especially in diabetics and pre-diabetics (3,6,32-34,37).
Abusive intakes of fat, in the morning or midday stages, interfere with hepatic metabolism causing FFA to be
increased in the blood. This leads to insulin resistance, resulting in the development of sustained
hyperglycemia during the rest of the day. In the case of high fat intakes at night, this can generate an
increase in unsaturated and pro-inflammatory fatty acids, mainly TRG, increasing the risk of developing
diseases related to blood vascularity. Among these are arteriosclerosis, dyslipidemia, stroke, and heart
disease (32-35,38).
In relation to protein intake, its poor practice can cause imbalances in the rhythms responsible for the
regulation of the mediator of amino acid mobilization during fasting (KLF15). That said, a high protein meal
at night is capable of causing metabolic alterations such as hypoglycemia, hyperammonemia, and even
deterioration in the urea cycle (31,39).
Carbohydrates in timing
Most studies (6,21,22) point out that the maximum peaks of carbohydrate expression occur in the early hours
of the morning, decreasing its tolerance with the passing of the day. This fact correlates, in some cases
and in others not, with insulin secretion (3,5,6,32,33).
A study conducted in 2017 by Kessler et al. (32) evaluated these glycemic responses by subjecting healthy
subjects to two types of diets separated in time. One group ingested a high carbohydrate (HC) meal in the
morning and a high fat (HF) meal in the afternoon (HC/HG), and another group did so in the reverse order
(HG/HF). After that, in subjects without impaired glucose tolerance, they observed that HC intakes in the
morning produced blood glucose levels with a higher peak expression compared to the afternoon. In addition,
after the afternoon intakes the levels persisted for longer periods of time as opposed to the morning where
they declined at a faster rate. Simultaneously, the same happened with insulin levels. In which the
response, sensitivity and secretion were lower in the later phases of the day.
Another study conducted in 2019 by Jamshed et al. (33) analyzed the effects of time-restricted feeding (TRF)
with 3 meals (breakfast, snack, and dinner) spread over 6 hours versus 3 meals spread over 12 hours (control
group). With respect to the first group, it was observed that, although glucose levels remained constant
without any variation during the period of intakes, there was a decrease during the resting phase. In the
second group (control group), their results showed that there was not much difference between the acrophases
of the different meals. They noted slightly higher glucose activity in the active phase in the morning than
in the resting phase in the afternoon, as was the case with insulin. In spite of this, they pointed out that
at breakfast the time of disappearance of the glucose peak was shorter and faster than at the rest of the
meals.
For another study developed in 2017 by Versteeg et al. (34) examined the influence of light on blood glucose
in diabetic and healthy subjects, simulating the phases of activity (morning) and rest (evening) using
different light intensities (bright light and dim light). As a result, they did not observe much variation
in glucose levels in healthy subjects in both light levels. Specifically, neither fasting nor postprandial
glucose and insulin levels were different in bright and dim light. In contrast to the previous studies,
diabetics showed an increase in glucose levels in bright versus dim light but little variation in the case
of insulin. Thus, they concluded that ambient light in the active phase can modify glucose levels in
diabetic subjects.
Another study conducted in 2018 by Takahashi et al. (6) examined the effect of timing (morning vs. afternoon)
of meal on postprandial glucose metabolism in healthy subjects. They were given a meal in the morning (9:00
h) after 10 hours of fasting and a meal in the afternoon (17:00 h) after 4 hours of fasting. In both times,
hardly any differences were observed. The only difference was that insulin levels were higher in the morning
than in the evening. While glycemic levels (Figure 13 A) were higher at night than in the morning.
Lipids in timing
As for the metabolic rhythms presented by lipids, almost in the same way as glucose, their peak of expression
in most types is higher during the morning, but in this case, prolonging until midday (3,35).
For the study conducted in 2017 by Kessler et al. (32), based on the intake of two types of diets (HC/HF)
distributed at different times of the day, it concluded that the HF diet did not show large differences in
the levels of free fatty acids between the different times of intake. However, a slight increase in free
fatty acids was observed throughout the day in subjects with glucose intolerance. Specifically, their peak
remained more pronounced during the afternoon stage. This generated an insulin resistance that further
decreased glucose tolerance as the day went on.
Likewise, in the study conducted in 2019 by Jamshed et al. (33), where the effects of TRF were analyzed
by ingesting 3 meals spread over different time intervals, it was observed that in both cases lipid
concentrations, in general, were more prominent during the day than at night, namely total cholesterol,
LDL, HDL, and FFA. Triglycerides (TRG) were the only exception that was elevated during the night.
Regarding the study developed in 2017 by Versteeg et al. (34) in which the influence of light on blood
glucose was examined in diabetic and healthy subjects, reflecting the phases of activity and rest, it
was noted that in healthy subjects exposure to bright light increased fasting and postprandial
concentrations of TRG but not that of FFA. In subjects with type 2 diabetes, TRG levels were also
increased by bright light exposure, although in this case even more so. For FFA, there was no
significant difference in the observed variations.
Moving away from these studies, which coincided in the analysis of glucose and lipids, we are faced with
other investigations related to lipid metabolism. One of them elaborated in 2018 by Poggiogalle et al.
(3) points out that HDL and LDL molecules show their highest levels around midday ranges. Being able to
establish 13:00 h as the average of these ranges. Another study carried out in 2015 by Sennels et al.
(36) highlights that the rhythms of TRG and diglycerides vary, showing peaks in the afternoon around
15:00 h and in the evening from 17:45-20:00 h.
Proteins in timing
About the relationship between the time of intake and the rhythms of protein activity, the study
conducted in 2018 by Takahashi et al. (6), which examined the effect of postprandial metabolic changes
between morning and evening meal in healthy subjects, found that some amino acids such as leucine,
lysine, histidine, tryptophan, arginine, asparagine, glutamic acid, glyceridic acid, or aspartic acid,
among others, described higher levels during the morning.
In another study conducted in 2012 by Jeyaraj et al. (31), where the circadian regulation of the protein
transcription factor KLF15 (mediator of fasting amino acid mobilization) was analyzed in mice, concluded
that a diet rich in protein severely altered metabolism by affecting this factor, presenting especially
blood levels of total amino acids, branched-chain amino acids (BCAAS), and urea more prominent at night.
Conclusions
Studies such as Takahashi, Kessler, or Jamshed (6,32,33) establish that the intake of carbohydrates
during the first hours of the day reflects maximum peaks of blood glucose expression compared to lower
levels produced in the final hours of the day. This in turn is accompanied by a faster rate of decline
from the peak. In addition, these glucose responses are reflected in insulin activity. Insulin levels
are lower at night than in the morning. This confirms a decrease in glucose tolerance as the day
progresses in relation to the low insulin activity towards the end of the day. However, in people who
are glucose intolerant (possible diabetics), glucose and insulin levels are even more increased at these
times of the day despite following the same pattern as in healthy subjects. In contrast to the above,
another study by Verteeg R et al. (34) notes that in both cases there are no differences between glucose
and insulin levels in healthy subjects. Again, only alterations and discrepancies appear among subjects
with diabetes, but in this case, glucose levels are even higher in the presence of daylight. All in all,
it is emphasized that nocturnal carbohydrate intake exacerbates glucose levels over time, producing
severe and long-lasting hyperglycemia in diabetes, prediabetes, and obesity due to the detriment of
insulin action (3,6,32).
Regarding lipid intake, studies such as that of Poggiogalle, Verteeg, or Sennels (3,34,36) state that
free fatty acids are the lipid molecules that present a greater affinity in their activity in the last
hours of the day. On the other hand, the study developed by Jamshed et al. (33) indicates that free
fatty acids present a better tolerance, due to a more efficient activity, during the first hours of the
day. On the other hand, the studies carried out by Poggiogalle, Jamshed, and Sennels (3,33,36) point out
that the TRG molecule presents a greater activity, again, in the final stages of the day. In contrast to
these, the study by Verteeg et al. (34) contradicts this, arguing that this occurs in the early stages.
In reference to the daily activity of total cholesterol, HDL and LDL, Jamshed et al. (33) are the only
ones who include these values in their assessment. They point out their greater tolerance towards hours
close to the morning and midday. In reference to people with glucose intolerance or predisposition
to it, the study carried out by Kessler et al. (32) points out that an intake rich in fats in the
morning increases FFA levels, maintaining them over time for several hours. And whose highest peaks are
generated in the evening as opposed to healthy subjects. This correlates with the development of insulin
resistance and thus decreased glucose tolerance, ultimately leading to nocturnal hyperglycemia. In
addition, the study by Verteeg et al. (34) further supports this, indicating that high nocturnal GRT
levels are further increased in this population group. Despite the inequality of results, all these
studies yield to the same fact. And it is that the poor hourly intake of lipids is related to the
increase of the probability for the development of arteriosclerosis and, with it, the drift to acute
conditions such as cerebrovascular accident, heart attack, and cardiopathies (32-35).
In relation to the time of protein intake and its effect/influence on its activity, only two studies were
found with conflicting results. On the one hand, Takahashi et al. (6) establish that the maximum levels
of certain postprandial amino acids (leucine, lysine, histidine, tryptophan, etc.) are produced in the
morning (6). While, on the other hand, Jeyaraj et al. (31) differ in this, highlighting that the rich
protein intake generates alterations in the transcription factor KLF15 inducing an increase of plasma
amino acids worsening in nocturnal stages. This generates an increase in the levels of total amino
acids, BCAAS and urea. At the same time, increasing the risk of the appearance of metabolic alterations
such as hypoglycemia, hyperammonemia, and even deterioration in the urea synthesis cycle (31,39).
All these contrasts of results between studies can be seen in the figure below (Figure 17)
(3,6,31,32,33,34,35,36):
However, as can be seen, the studies cited throughout this review present conflicting results that limit
the development of clear and reliable conclusions regarding the knowledge on the relationship between
the time of intake and its effect on the metabolism of the main nutrients. Divergences attributed to one
or more factors but mainly to the design of each study. Since each one presents different inclusion and
exclusion criteria, different time periods (most of them short term, something unfavorable for obtaining
consistent data), different intervention methods (in some cases, the diets were not well defined or
adapted to each subject, or there was an absence of nutritional advice), as well as the methods and
instruments used in data collection and analysis.
With all this, we conclude that the time of intake is a determinant on the activity and response of the
main nutrients, as it exerts an effect on the synchronization that metabolic processes maintain with the
circadian system. Most of the results agree that the maximum rhythms of glucose expression, and
therefore of activity, occur during the first hours of the day. The tolerance, due to the decrease in
insulin activity, is abandoned as the day progresses. As for lipid intake, its rhythms of expression are
more prominent in nocturnal stages, declining in the morning. Regarding the rhythms of protein/amino
acid expression, there are no conclusive data due to the lack of data for their study. All these results
seem to be logical since the first hours of the day are related to the most active phase of the
organism. In spite of this, the objective of this research must be studied in greater depth in order to
have more exact and reliable information, which can be transferred and applied for the knowledge of the
maintenance, improvement, and/or prevention of the health of the human being.
References
(1) Panda S. Circadian physiology of metabolism. Science. Nov 25, 2016;354(6315):1008-15. Available at:
10.1126/science.aah4967
(2) McKenna H, van der Horst GTJ, Reiss I, Martin D. Clinical chronobiology: a timely consideration in
critical care medicine. Crit Care. May 11, 2018;22(1):124. Available at:
10.1186/s13054-018-2041-x
(3) Poggiogalle E, Jamshed H, Peterson CM. Circadian regulation of glucose, lipid, and energy metabolism
in humans. Metabolism. July 2018;84:11-27. Available at: 10.1016/j.metabol.2017.11.017
(4) Johnston JD, Ordovás JM, Scheer FA, Turek FW. Circadian Rhythms, Metabolism, and Chrononutrition in
Rodents and Humans123. Adv Nutr. Mar 9, 2016;7(2):399-406. Available at: 10.3945/an.115.010777
(5) Maury E. Off the Clock: From Circadian Disruption to Metabolic Disease. Int J Mol Sci. Mar 30,
2019;20(7):E1597. Available at: 10.3390/ijms20071597.
(6) Takahashi M, Ozaki M, Kang M-I, Sasaki H, Fukazawa M, Iwakami T, et al. Effects of Meal Timing on
Postprandial Glucose Metabolism and Blood Metabolites in Healthy Adults. Nutrients. Nov 14,
2018;10(11):E1763. Available at: 10.3390/nu10111763.
(7) Kessler K, Hornemann S, Rudovich N, Weber D, Grune T, Kramer A, et al. Saliva Samples as A Tool to
Study the Effect of Meal Timing on Metabolic And Inflammatory Biomarkers. Nutrients. Jan 28,
2020;12(2):340. Available at: 10.3390/nu12020340.
(8) Montaruli A, Castelli L, Mulè A, Scurati R, Esposito F, Galasso L, Roveda E. Biological Rhythm and
Chronotype: New Perspectives in Health. Biomolecules. Mar 24, 2021;11(4):487. Available
at: 10.3390/biom11040487.
(9) Oosterman JE, Kalsbeek A, la Fleur SE, Belsham DD. Impact of nutrients on circadian rhythmicity. Am
J Physiol Regul Integr Comp Physiol. 2015 Mar 1;308(5):R337-350. Available at:
10.1152/ajpregu.00322.2014
(10) Mazri FH, Manaf ZA, Shahar S, Mat Ludin AF. The Association between Chronotype and Dietary Pattern
among Adults: A Scoping Review. Int J Environ Res Public Health. Dec 20, 2019;17(1):68. Available
at: 10.3390/ijerph17010068
(11) Dibner C, Schibler U. Circadian timing of metabolism in animal models and humans. J Intern Med. May
2015;277(5):513-27. Available at: 10.1111/joim.12347.
(12) Weger BD, Gobet C, David FPA, David FPA, Atger F, Martin E, Phillips NE, Charpagne A, Weger M, Naef
F, Gachon F. Systematic analysis of differential rhythmic liver gene expression mediated by the
circadian clock and feeding rhythms. Proc Natl Acad Sci U S A. Jan 19,
2021;118(3):e2015803118. Available at: 10.1073/pnas.2015803118
(13) Moreno C. The recognition of Chronobiology in Science. Sleep Sci. Feb. 2018;11(1):1.
Available at: 10.5935/1984-0063.20180001
(14) Sehgal A. Physiology Flies with Time. Cell. Nov 30, 2017;171(6):1232-5. Available at:
10.1016/j.cell.2017.11.028
(15) Mukherji A, Bailey SM, Staels B, Baumert TF. The circadian clock and liver function in health and
disease. J Hepatol. July 2019;71(1):200-211. Available at: 10.1016/j.jhep.2019.03.020
(16) Partch CL, Green CB, Takahashi JS. Molecular Architecture of the Mammalian Circadian Clock. Trends
Cell Biol. 2014 Feb;24(2): 90-9. Available at: 10.1016/j.tcb.2013.07.002
(17) Roenneberg T, Merrow M. The Circadian Clock and Human Health. Curr Biol. 2016 May
23;26(10):R432-443. Available at: 10.1016/j.cub.2016.04.011
(18) Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev
Genet. March 2017;18(3):164-79. Available at: 10.1038/nrg.2016.150.
(19) Paoli A, Tinsley G, Bianco A, Moro T. The Influence of Meal Frequency and Timing on Healt in Humans:
The Role of Fasting. Nutrients. April 2019; 11(4): 719. Available at: 10.3390/nu11040719.
(20) Shaw E, Leung GKW, Jong J, Coates AM, Davis R, Blair M, et al. The impact of time of day on Energy
Expenditure: Implications for Long-Term Energy Balance. Nutrients. Oct
6, 2019;11(10):2383. Available at: 10.3390/nu11102383.
(21) Chauchan R, Chen K-F, Kent BA, Crowther DC. Central and peripheral circadian clocks and their role
in Alzheimer's disease. Dis Model Mech. Oct 1, 2017; 10(10): 1187-99. Available at:
10.1242/dmm.030627.
(22) Kumar Jha P, Challet E, Kalsbeek A. Circadian rhythms in glucose and lipid metabolism in nocturnal
and diurnal mammals. Mol Cell Endocrinol. Dec 15, 2015;418 Pt 1:74-88. Available at:
10.1016/j.mce.2015.01.024
(23) Albrecht, U. The circadian clock, metabolism and obesity. Obesity
Reviews. February 2017;18 Suppl, 1:25-33. Available at: 10.1111/obr.12502.
(24) Li MD, Ruan HB, Hughes ME, Lee JS, Singh JP, Jones SP, Nitabach MN, Yang X. O-GlcNAc signaling
entrains the circadian clock by inhibiting BMAL1/CLOCK ubiquitination. Cell Metab. Feb 5,
2013;17(2):303-10. Available at: 10.1016/j.cmet.2012.12.015
(25) Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, Nusinow DA, Sun X, Landais S, Kodama Y,
Brenner DA, Montminy M, Kay SA. Cryptochrome mediates circadian regulation of cAMP signaling and
hepatic gluconeogenesis. Nat Med. Oct 1, 2010;16(10):1152-6. Available at:
10.1038/nm.2214
(26) Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome
regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci U S A. July
31, 2012;109(31):12662-7. Available at: 10.1073/pnas.1209965109
(27) Shi L, Tu BP. Acetyl-CoA and the regulation of metabolism: mechanisms and consequences. Curr Opin
Cell Biol. Apr 2015;33:125-31. Available at: 10.1016/j.ceb.2015.02.003
(28) Hussain MM, Pan X. Circadian Regulation of Macronutrient Absorption. J Biol Rhythms. 2015 Aug
12;30(6):459-69. Available at: 10.1177/0748730415599081
(29) Neufeld-Cohen A, Robles MS, Aviram R, Manella G, Adamovich Y, Ladeuix B, Nir D, Rousso-Noori L,
Kuperman Y, Golik M, Mann M, Asher G. Circadian control of oscillations in mitochondrial
rate-limiting enzymes and nutrient utilization by PERIOD proteins. Proc Natl Acad Sci U S
A. Mar 22, 2016;113(12):E1673-82. Available at: 10.1073/pnas.1519650113
(30) Adamovich Y, Rousso-Noori L, Zwighaft Z, Neufeld-Cohen A, Golik M, Kraut-Cohen J, Wang M, Han X,
Asher G. Circadian clocks and feeding time regulate the oscillations and levels of hepatic
triglycerides. Cell Metab. 2014 Feb 4;19(2):319-30. Available at:
10.1016/j.cmet.2013.12.016
(31) Jeyaraj D, Scheer FAJL, Ripperger JA, Haldar SM, Lu Y, Prosdocimo DA, et al. Klf15 orchestrates
circadian nitrogen homeostasis. Cell Metab. Mar 7, 2012;15(3):311-23. Available at:
10.1016/j.cmet.2012.01.020
(32) Kessler K, Hornemann S, Petzke KJ, Kemper M, Kramer A, Pfeiffer AFH, et al. The effect of diurnal
distribution of carbohydrates and fat on glycaemic control in humans: a randomized controlled trial.
Sci Rep. Mar 8, 2017;7:44170. Available at: 10.1038/srep44170.
(33) Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early Time-Restricted Feeding
Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in
Humans. Nutrients. May 30, 2019;11(6):E1234. Available at: 10.3390/nu11061234.
(34) Versteeg RI, Stenvers DJ, Visintainer D, Linnenbank A, Tanck MW, Zwanenburg G, et al. Acute Effects
of Morning Light on Plasma Glucose and Triglycerides in Healthy Men and Men with Type 2 Diabetes. J
Biol Rhythms. 2017 Mar 20;32(2):130-42. Available at: 10.1177/0748730417693480
(35) Gooley JJ. Circadian regulation of lipid metabolism. Proc Nutr Soc. May 26, 2016
Nov;75(4):440-50. Available at: 10.1017/S0029665116000288
(36) Sennels HP, Jørgensen HL, Fahrenkrug J. Diurnal changes of biochemical metabolic markers in healthy
young males - the Bispebjerg study of diurnal variations. Scand J Clin Lab Invest. Sep
17, 2015;75(8):686-92. Available at: 10.3109/00365513.2015.1080385
(37) Haldar S, Egli L, De Castro CA, Tay SL, Koh MXN, Darimont C, et al. High or low glycemic index (GI)
meals at dinner results in greater postprandial glycemia compared with breakfast: a randomized
controlled trial. BMJ Open Diabetes Res Care. 2020 Apr;8(1):e001099. Available at:
10.1136/bmjdrc-2019-001099
(38) Maugeri A, Vinciguerra M. The Effects of Meal Timing and Frequency, Caloric Restriction, and Fasting
on Cardiovascular Health: an Overview. J Lipid Atheroscler. Jan
15, 2020;9(1):140-52. Available at: 10.12997/jla.2020.9.1.140
(39) Smeuninx B, Greig CA, Breen L. Amount, Source and Pattern of Dietary Protein Intake Across the Adult
Lifespan: A Cross-Sectional Study. Front Nutr. March 16, 2020;7:25. Available at:
10.3389/fnut.2020.00025